Product
Motion Bioreactor Bags
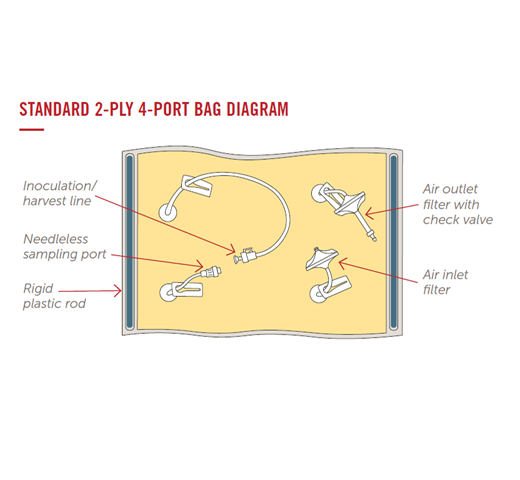
Entegris Motion Bioreactor Bioprocess Bags are safe and secure single, double and triple barrier cell culture bags. Starting with the preferred motion bioreactor, end users can choose the bag film, bag size, port geometry, port quantity, port location, tubing material, connector type, and more from Entegris’ extensive library of standard bioprocessing materials and components.
In addition, Entegris motion bioreactor bags are manufactured to the most exacting standards. To assure performance the customer can rely on, quality control inspections are performed from the raw material state to the final product. The entire process occurs under a quality system compliant with FDA standards.